














上海市浦东新区凯庆路59号12幢(西区)

上海市浦东新区仁庆路509号7号楼(东区)
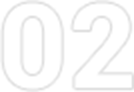


收到邮件后,我们将会第一时间回复您

版权所有 © Copyright 2025 上海泓博智源医药股份有限公司【官网】 隐私政策 现代奴隶制声明
备案号:沪ICP备20013779号 公安备案:沪公网安备31011502403341号 技术支持:澳煦互动
